
ΑΙhub.org
A deep learning pipeline for controlling protein interactions
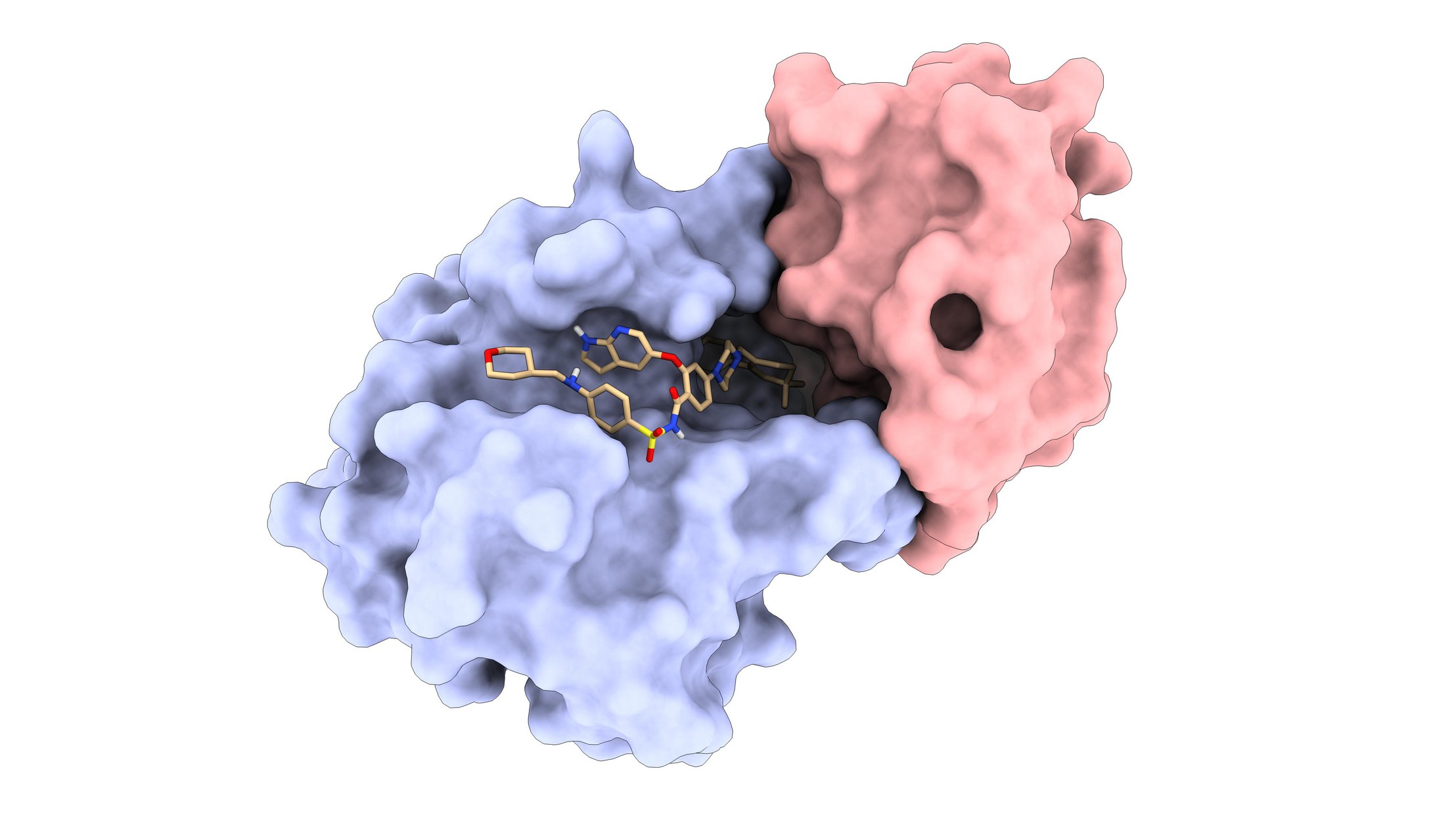 One of the LPDI’s de novo protein binders (red) bound to the protein Bcl2 (blue) in complex with FDA-approved drug Venetoclax (beige) © LPDI EPFL
One of the LPDI’s de novo protein binders (red) bound to the protein Bcl2 (blue) in complex with FDA-approved drug Venetoclax (beige) © LPDI EPFL
By Celia Luterbacher
In 2023, scientists in the joint School of Engineering and School of Life Sciences Laboratory of Protein Design and Immunoengineering (LPDI), led by Bruno Correia, published a deep-learning pipeline for designing new proteins to interact with therapeutic targets. MaSIF can rapidly scan millions of proteins to identify optimal matches between molecules based on their chemical and geometric surface properties, enabling scientists to engineer novel protein-protein interactions that play key roles in cell regulation and therapeutics.
A year and a half later, the team has reported an exciting advancement of this technology. They have used MaSIF to design novel protein binders to interact with known protein complexes involving small molecules like therapeutic drugs or hormones. Because these bound small molecules induce subtle changes in the surface properties (‘neosurfaces’) of these protein-drug complexes, they can act as ‘on’ or ‘off’ switches for the fine control of cellular functions like DNA transcription or protein degradation.
“Our idea was to engineer an interaction in which a small molecule causes two proteins to come together. Some approaches have focused on screening for such small molecules, but we wanted to design a novel protein that would bind to a defined protein-drug complex,” says LPDI scientist and co-first author Anthony Marchand.
Remarkably, the team showed that MaSIF could seamlessly apply protein surface representations (‘fingerprints’) that had been trained only on proteins to neosurfaces emerging from protein-drug complexes. While most learning-based protein design systems work only on amino acid building blocks from nature, MaSIF’s sensitivity and generalizability to small molecules means it could be used to design chemically induced protein interactions in engineered cells for drug-controlled cell-based therapies or biosensors.
If you can precisely control the spatiotemporal activity of cell-based therapies with small molecule switches, then you can really improve the safety and efficacy of the treatment.
– Stephen Buckley, LPDI EPFL
Small but powerful
While protein binding may seem as simple as fitting puzzle pieces together, in reality, protein surface variations make it hard to predict how and where binding events will occur. As in their previous study, the team designed novel protein binders by using MaSIF to generate ‘fingerprints’ for surface features like positive and negative charge, hydrophobicity, shape, etc. Then they identified complementary surfaces from a database, digitally grafted protein fragments onto larger scaffolds, and selected binders predicted to fit best with their targets.
“The difference here is that we assume the surface features of a protein change if a small molecule binds to it, creating a neosurface. MaSIF was able to capture this difference with a high degree of sensitivity,” says LPDI PhD student and co-author Arne Schneuing.
The team experimentally validated their novel protein binders against three drug-bound protein complexes containing the hormone progesterone, the FDA-approved leukemia drug Venetoclax, and the naturally occurring antibiotic Actinonin, respectively. The protein binders designed using MaSIF successfully recognized each drug-protein complex with high affinity. The researchers explain that this was possible because MaSIF is based on general surface features that apply to proteins and small molecules alike, so they were able to map the small molecule features onto the same descriptor space that MaSIF was trained on for proteins.
“MaSIF has a relatively small number of parameters – around 70,000 versus billions for large deep learning systems like ChatGPT. This is possible because we use only key surface features, resulting in a high level of abstraction. In other words, we don’t give the system the full picture; only the part we think matters for solving the problem,” Schneuing says.
Better control of CAR-T cells
An exciting potential application of this work is the fine control of cell-based cancer treatments like chimeric antigen receptor (CAR-T) therapy, which involves engineering a patient’s T cells to better target their cancer. But after being re-introduced into the patient, engineered cells may attack the wrong targets – potentially causing harmful side-effects – or may exhaust their ability to fight cancer. In a proof-of-concept experiment, the EPFL team showed that a Venetoclax-inducible system designed with MaSIF was effective at switching on tumor killing activity of CAR-T cells in vitro.
“If you can precisely control the spatiotemporal activity of cell-based therapies with small molecule switches, then you can really improve the safety and efficacy of the treatment,” summarizes LPDI PhD student and co-first author Stephen Buckley.
This work was carried out in collaboration with Michael Bronstein, Scientific Director of the Research Institute for Biomedical Artificial Intelligence (AITHYRA) of the Austrian Academy of Sciences.
References
- Targeting protein–ligand neosurfaces with a generalizable deep learning tool, Marchand, A., Buckley, S., Schneuing, A. et al. Nature (2025).
- De novo design of protein interactions with learned surface fingerprints, Gainza, P., Wehrle, S., Van Hall-Beauvais, A. et al. Nature (2023).









