
ΑΙhub.org
Artificial intelligence helps elucidate the precise spatial structure of complex chiral molecules
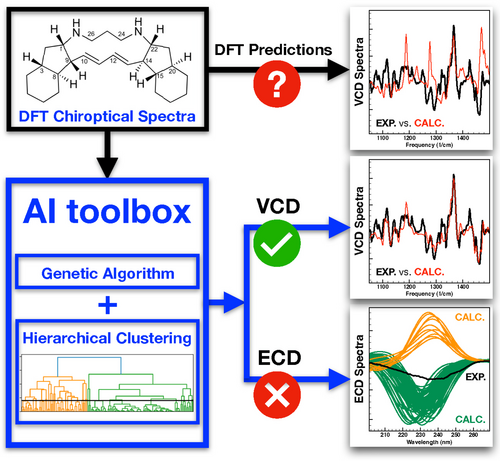 Chiroptical spectroscopies are key to determine the absolute configuration of solvated compounds. Combining a genetic algorithm and a hierarchical clustering analysis enables a major improvement of the analysis of chiroptical spectra and the reliability of the assignment. Image from An Artificial Intelligence Approach for Tackling Conformational Energy Uncertainties in Chiroptical Spectroscopies, reproduced under a CC BY 4.0 licence.
Chiroptical spectroscopies are key to determine the absolute configuration of solvated compounds. Combining a genetic algorithm and a hierarchical clustering analysis enables a major improvement of the analysis of chiroptical spectra and the reliability of the assignment. Image from An Artificial Intelligence Approach for Tackling Conformational Energy Uncertainties in Chiroptical Spectroscopies, reproduced under a CC BY 4.0 licence.
Researchers from the University of Amsterdam’s Van ’t Hoff Institute for Molecular Sciences have developed a powerful machine learning approach to elucidate the absolute configuration and conformational landscape of complex chiral molecules. In a recent paper in Angewandte Chemie, they describe how the combination of a genetic algorithm with a hierarchical clustering algorithm can predict and boost the performance of chiroptical spectroscopies. The power of this approach is demonstrated in a study together with researchers from the National Institutes of Health (USA) on natural product alkaloids isolated from marine sponges. Containing as many as eight chiral centres, an unambiguous determination of their stereochemistry is key for understanding their biosynthetic pathways.
Determining the precise spatial structure of a chiral molecule, the absolute configuration, is far from easy. The common approach is to use circularly polarized light that itself displays chirality. Techniques such as electronic circular dichroism (ECD), vibrational circular dichroism (VCD) and Raman optical activity (ROA) spectroscopy apply such light to yield a spectroscopic fingerprint. This is unique for each molecule and can even distinguish the mirror image of the same molecule.
However, to derive an absolute configuration from a recorded spectrum, a comparison with simulated spectra is needed. The latter can be generated, for example, using Density Functional Theory (DFT), but for complex molecules in particular this has serious limitations. Firstly, the simulation can become time-consuming and computationally expensive. Secondly, for such a comparison, an average needs to be taken over all possible conformations the molecule can adopt. And here comes the snag, because this average critically depends on the energies of these conformers. As there is an intrinsic uncertainty in these energies, there can be a large variation in simulated spectra. The larger the number of chiral centres and the larger the conformational flexibility, the poorer the quality of the stereochemical determination.
Reliable assignment of absolute configuration
In their Angewandte paper, the researchers describe how the strategic use of artificial intelligence helps to overcome this impasse. They present an elaborate protocol that combines two artificial intelligence (AI) algorithms. The first is a genetic algorithm that explicitly takes the uncertainties in the computed energies into account in the comparison between experimental and predicted spectra to help identify the most relevant conformations out of all possible conformations. The second is a hierarchical clustering algorithm that analyses the trends in the spectra of the considered conformations. It also identifies on-the-fly when a given chiroptical technique is not able to make any reliable conformational predictions.
The paper shows how the novel “AI toolbox” leads to reliable assignments of absolute configuration and provides insight into conformational heterogeneities – even for difficult molecules that are flexible and feature a large number of chiral centres. According to the researchers, their approach is quite general and applicable to the analysis of any type of molecular spectra. They expect their approach to improve the analysis of spectra of complex systems in general.
A relevant point-in-case
As a case study the researchers applied their protocol to two natural products isolated from the marine sponge Haliclona. The compounds papuamine and haliclonadiamine always occur together in the sponges, and determination of their absolute configuration is key for establishing their exact biosynthetic pathway. Both molecules are very flexible, have very similar structures and share eight chiral centres. Earlier research by the Laboratory of Bioorganic Chemistry at the National Institutes of Health (Bethesda, US) has shown that it is very difficult to unambiguously determine their configuration with current approaches. Papuamine and haliclonadiamine therefore were the ideal examples for illustrating the weakness of the standard protocol and the effectiveness of the newly proposed one, enabling highly reliable assignments of their absolute configurations.
Read the work in full
An Artificial Intelligence Approach for Tackling Conformational Energy Uncertainties in Chiroptical Spectroscopies, Gabriel Marton, Mark A. J. Koenis, Hong-Bing Liu, Carole A. Bewley, Wybren Jan Buma, Dr. Valentin Paul Nicu, Angewandte Chemie (2023).










